electron dot structure of h2s|electron dot structure of f2 : Manila H2S Lewis Structure - How to Draw the Dot Structure for H2S. Watch on. 6 Steps to Draw the Lewis Structure of H2S. Step #1: Calculate the total number of valence electrons. .
With our Free Poker Money No Deposit Offers (also known as "Instant Bankroll"), we will give you free poker money to test your skills at the real money tables. No credit card is required. No bank account information. No gimmicks. Signing up for free poker money is as easy as 1,2,3. Since 2004, we have funded thousands of online poker accounts .
PH0 · shape of h2s
PH1 · electron dot structure of hydrogen
PH2 · electron dot structure of f2
PH3 · electron dot structure of ethanoic acid
PH4 · draw the electron dot structures for h2s
PH5 · Iba pa
Perfect for anyone who wants a digital pilot logbook. Excel Pilot Logbook ™ is the most durable and future-proof electronic pilot logbook. It’s a universally accessible format for life, editable, and easily customized. Put it on Google Drive (or any other file-sharing app), and it’s in the cloud and backed up for free.
electron dot structure of h2s*******A step-by-step explanation of how to write the Lewis Dot Structure for H2S (Dihydrogen Sulfide). The H2S Lewis structure is similar to the structure for water H2O since Sulfur (S) and (O) are.
The Lewis structure of H2S is similar to H2S. Sulfur needs eight electrons to fulfill the requirements for Octet Rule. But Hydrogen only requires a single electron to become .
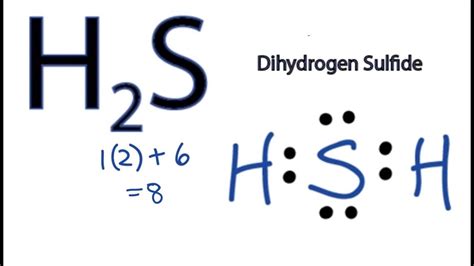
It’s good to know that lewis structure is all about electrons and atoms fulfilling their octet. Every atom tries to fulfill its octet to attain stability. In this compound, sulfur has 6 .371. 40K views 2 years ago Lewis Structures. Hydrogen and sulfur are both non-metals, and so they SHARE electrons to form covalent bonds (this makes it a covalent aka .Total number of electrons of the valance shells of H 2 S. There are two elements; hydrogen and sulfur. Hydrogen is a group IA element and has only one electron in its last shell .H2S Lewis Structure - How to Draw the Dot Structure for H2S. Watch on. 6 Steps to Draw the Lewis Structure of H2S. Step #1: Calculate the total number of valence electrons. .
Draw the Lewis dot structure of Hydrogen sulphide molecule . Doubtnut. 3.15M subscribers. Subscribed. 204. 13K views 3 years ago. Draw the Lewis dot structure of .
electron dot structure of h2sWhat is the Lewis structure of hydrogen sulfide H2S? Hydrogen sulfide H 2 S is a gas with a foul smell, often described as being similar to rotten eggs. It is composed of two .Determining the Total Valence Electrons. To accurately represent the H2S Lewis structure, we need to calculate the total valence electrons. Sum the valence electrons .Step-1: H2S Lewis dot Structure by counting valence electrons on the sulfur atom. Step-2: Lewis Structure of H2S for counting valence electrons around the terminal hydrogen .
Step 5: Check the octet rule (formal charges) In the Lewis structure of hydrogen sulfide, sulfur has eight electrons around it, including two lone pairs and two bonding pairs. Each hydrogen atom has two electrons around it. The formal charge is a measure of the distribution of electrons in a molecule. It is calculated by subtracting the .electron dot structure of f2To sketch the H2S Lewis structure by following these instructions: Step-1: H2S Lewis dot Structure by counting valence electrons on the sulfur atom. Step-2: Lewis Structure of H2S for counting valence electrons .Hydrogen sulfide (H2S) consists of two hydrogen (H) atoms and one sulfur (S) atom. Sulfur is located in group 16 of the periodic table, indicating that it has six valence electrons, while hydrogen belongs to group 1 and brings one valence electron per atom. Step-by-Step Guide to Drawing the Lewis Structure of H2S 1. Determining
The Lewis structure of hydrogen sulfide is best represented as a bent H{eq}_2 {/eq}S molecule with two lone pairs of electrons on the S atom represented by two pairs of dots (or two bars). The . Total electron pairs = total valence electrons ÷ 2. So the total electron pairs = 8 ÷ 2 = 4. Third, determine the central atom; Here hydrogen can not be the central atom. Because the central atom is bonded with at least two other atoms, and hydrogen has only one electron in its last shell, so it can not make more than one bond. Now in the H2S molecule, you have to put the electron pairs between the sulfur atom (S) and hydrogen atoms (H). This indicates that the sulfur (S) and hydrogen (H) are chemically bonded with each other in a H2S molecule. Step 4: Make the outer atoms stable. Place the remaining valence electrons pair on the central atom. Answer: To draw the lewis dot structure of H₂S, we have to find out the valence electrons of sulfur and hydrogen first.We express valence electrons as dots in lewis dot structure. . The nunber of valence electrons in sulfur is 6. Again, we have to find out the valence electrons of hydrogen,H.
H2S Lewis Structure. According to the H2S of the valence, electrons and nonbonding electron pairs participate in bond formation. Knowing the Lewis structure of a chemical compound is crucial since it provides information on all the substance’s other chemical properties. Dots and lines in the illustration represent the electrons.
electron dot structure of H2S class 10. by Remedial Classes on September 30, 2023 in Class10. In this article, we are going to discuss the electron dot structure of H 2S H 2 S which is also known as the Lewis structure of H 2S H 2 S. We will discuss the electron dot structure of H 2S H 2 S through the following points. 1.Let's do the Lewis structure for H2S: Dihydrogen Sulfide. On the periodic table: Hydrogen, group 1, has 1 valence electron, but we have two Hydrogens here so let's multiply that by 2. Plus Sulfur is in group 6 or 16 on the periodic table, so it has 6 valence electrons. Total of 8 valence electrons. Let's draw this thing. We'll put Sulfur here. A hydrogen atom is shown as H⋅ H ⋅ because of its one valence electron. The structures of molecules that are held together by covalent bonds can be diagrammed by Lewis electron-dot structures. The hydrogen molecule is shown in the figure below. Figure 9.5.2 9.5. 2: On the left is a single hydrogen atom with one electron.
What is the Lewis dot structure of hydrogen sulfide (H 2 S)? The Lewis dot structure of H 2 S displays a total of 8 valence electrons, i.e., 4 electron pairs. A sulfur (S) atom is present at the center. It is .Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence .Draw electron dot structure of H2S ( hydrogen sulphide) , class -10thlinks for other previous video👇👇word root, suffix, prifix :-https://youtu.be/AxDGfJrn0.GENERAL TERMS FOR LEWIS DOT STRUCTURES: 1. Dot • one dot represents one valence electron (found on odd-electron particles). 2. Pair of Dots •• a pair of dots represents a nonbonding (lone) pair of electrons that are not involved in a covalent bond and "belong to" only one atom. 3. Dash each dash represents two electrons that are .
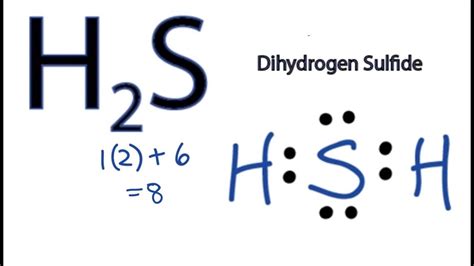
The article describes the H2S lewis structure with other properties it has which can be describe from the hybridization of the structure and the ‘d’ orbital that Sulfur carries. . the angle between the two nonbonding electron pairs increases for stabilizing the electron dot structure from the repulsion of dense lone electron pairs .Q. (a) Draw the electron dot structure for: (a) ethanoic acid. (b) Draw the electron dot structure for: H2S. (c) Draw the electron dot structure for: propanone. (d)
The Lewis structure of H2S is based on the number of total valence electrons present in sulphur and hydrogen atoms. (c) Propanone. The simplest and smallest ketone, propanone, is chemically defined as (CH3)2CO, and it is the most common substance called “acetone.”. (d) F2. An electron is represented by a dot. One electron is needed to .
Kapuso fam! Sa pagbabalik ng 'Family Feud,' simula na rin muli ng pamimigay ng papremyo sa 'Guess To Win' promo ng programa. Ang buong detalye, panoorin sa video na ito. Tutukan ang season comeback ng 'Family Feud,' Simula October 2, .
electron dot structure of h2s|electron dot structure of f2